Unstoppable good news for medical firms in BDA
Jacobio completes the first patient administration in the SHP2 combination trial
Jacobio Pharmaceutical announced that the company's research project SHP2 inhibitor JAB-3312 has completed clinical trials and is ready for the first patient to receive the drug with PD-1 antibody Pembrolizumab and MEK inhibitor Binimetinib.
After that, Jacobio will receive a milestone payment of $20 million from its partner AbbVie.
Jacobio Pharmaceutical Group Co,. Ltd. is committed to developing allosteric inhibitors against protein tyrosine phosphatase, KRAS and transcriptional factors. [Photo/kfqgw.beijing.gov.cn]
Jacobio and AbbVie signed a cooperation agreement in May 2020.
The two parties will jointly develop the small molecule anti-tumor SHP2 inhibitors (including JAB-3068 and JAB-3312) independently developed by Jacobio.
Jacobio is conducting SHP2 inhibitor single-drug and combination trials in more than 30 clinical centers around the world.
Genetron Health and Liaoning Cancer Hospital jointly establish a key laboratory of precision medicine for malignant tumors
Genetron Health and Liaoning Cancer Hospital signed a cooperation agreement for the Key Laboratory of Precision Medicine for Malignant Tumors at the Science and Technology Conference of the Affiliated Cancer Hospital of Dalian University of Technology on June 16, 2021.
At the meeting, Wang Danbo, superintendent of Liaoning Cancer Hospital and Dong Lei, senior marking director of Genetron Health, signed a cooperation agreement for the laboratory and inaugurated its nameplate.

Wang Danbo and Dong Lei unveil the nameplate of the Key Laboratory of Precision Medicine for Malignant Tumors on June 16. [Photo/kfqgw.beijing.gov.cn]
About 260 people, including Song Yongchen, vice president of Dalian University of Technology, Teng Lin, vice president of Genetron Health, and other hospital staff attended the conference.
Betta Pharmaceutical's BPI-16350 drug combination was approved to carry out clinical trials
Betta Pharmaceuticals Co., Ltd. received the "Notice of Drug Clinical Trial Approval" issued by the National Medical Products Administration (Notice No.: 2021LP00866, 2021LP00867).
The company declared that BPI-16350 capsules combined with non-steroidal aromatization enzyme inhibitors (letrozole/anastrozole), human epidermal growth factor receptor 2 negative (HR positive/HER2 negative) for advanced breast cancer phase Ib/II clinical trial application have been approved by the National Medical Products Administration.
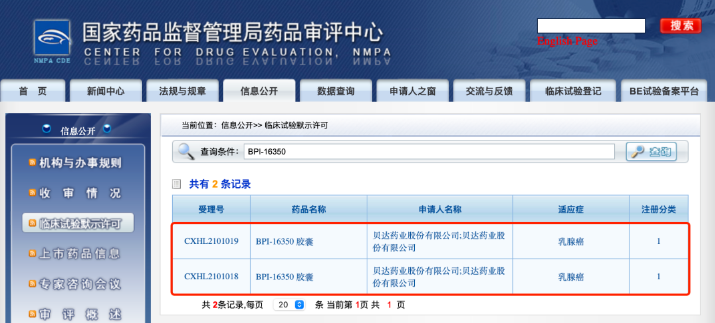
The National Medical Products Administration approves the clinical trials of Betta Pharmaceutical's BPI-16350 drug combination. [Photo/kfqgw.beijing.gov.cn]
BPI-16350 is a new molecular entity compound with completely independent intellectual property rights.
It was independently developed by Betta Pharmaceuticals for the treatment of HR-positive/HER2-negative advanced or metastatic breast cancer patients. It may also be used for Rb+ first-line, second-line or as a combination therapy for other cancers.

