A breakthrough in biotechnology and big health Industry
With the assistance of the composite master-slave orthopedic endoscopic surgical robot developed by Great Robotics, a company located in Beijing E-Town, several top orthopedic experts in China completed the world's first 5G remote orthopedic endoscopic surgical robot research surgery.
Building on the initial research surgery conducted this March, they have made breakthroughs in various new procedures, achieving significant innovation and advancement.
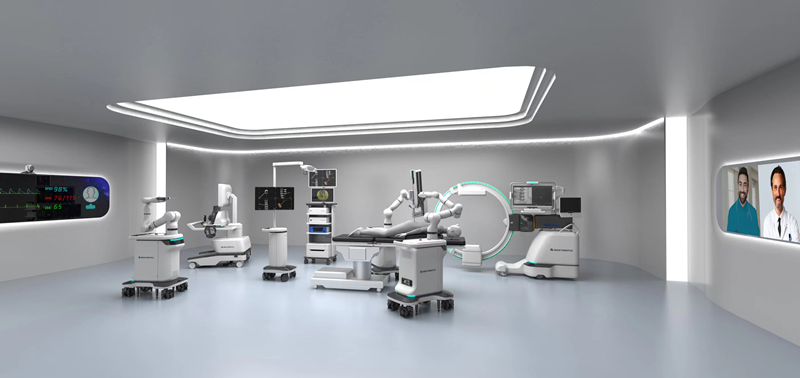
A view of the operating room [Photo/beijingetown.com.cn]
Due to technological limitations in the past, 5G remote orthopedic surgeries mainly involved remote consultations, surgical planning, or navigation positioning. However, this surgical robot system has truly realized the entire process of orthopedic minimally invasive 5G remote-controlled surgery.
It includes remote image acquisition and processing, remote surgical planning, remote automatic guidance, as well as remote master-slave direct control of the surgical robot to complete all core surgical steps. In this process, high-precision intraoperative imaging is obtained through the intraoperative mobile imaging system independently developed by Great Robotics.
This is the world's first fully electric 8-axis integrated precise intraoperative imaging robot, which has a scanning speed of 12 seconds, image accuracy of 0.16 millimeters, an intelligent AGV robot chassis, and a fully electric wireless integrated design.
The imaging robot serves as both a high-precision imaging equipment developed specifically for the surgical robot system and can be widely used in various robotic surgeries, navigation robot surgeries, manual navigation surgeries, as well as various minimally invasive, interventional, and open surgeries.
While fully leveraging the leading enterprises' aggregation effect, Beijing E-Town continues to support the transformation of innovative achievements and has gathered a group of internationally renowned entrepreneurs and scientific teams for entrepreneurship and development in Beijing.
Recently, Evive Biotech, an enterprise in Beijing E-Town, announced that its macromolecular new medicine has received approval from the National Medical Products Administration (NMPA) for listing.
This medicine is used to prevent and treat neutropenia in tumor patients after receiving chemotherapy drugs, making it the first domestically approved third-generation long-acting G-CSF. According to incomplete statistics, nearly 100 innovative medicines and medical devices are expected to be in clinical and approval stages in Beijing E-Town in 2023.

